When
I tell friends that I conduct research at the Smithsonian, most think
immediately of Washington. Fellow students and I are currently enrolled
in a tropical biology field course at the Smithsonian... in Panamá, not
not on the Potomac shoreline! So let’s make things clear with a quick
overview (i.e. publicity shpiel) of STRI, one of the world’s flagships
of tropical research.
The
Smithsonian Tropical Research Institute (STRI) is a community of
researchers and scholars interested in the tropics. It is part of the
Smithsonian Institution network and hosts 40 permanent scientists, 400
support staff and 1,400 visiting scientists and students. My colleagues
and I, all graduate students of the University of Illinois at
Urbana-Champaign, the Instituto de Investigaciones Científicas y Servicios de Alta Tecnología (INDICASAT) and McGill University’s NEO program, are part of this community.
Together,
we seek to understand the tropics, in all their complexity, and merge
our diverse areas of expertise to do so. According to STRI’s Scientist
Emeritus, Egbert Leigh Jr., most of STRI’s research can be grouped under
12 broad areas. First, we seek to contrast and compare two oceans, the
Pacific and the Atlantic, and understand how they came to be so
different. We try to accumulate as much data as possible on the recent
past, to understand what is happening today in both the human and
natural worlds. We seek to understand the distant past through
archaeology, and learn how our world came to be. We try to uncover why
and how individuals diverge within a species to give rise to more
species. We try to unravel the mysteries of mutualism, or why some
species collaborate with each other while others prefer to cheat. We
study social behaviour in animals, but also in humans within the Central
American context. We want to understand what natural selection favors
and why some traits make it to the next generation while others do not.
We study the factors regulating populations of living organisms and the
inner workings of food webs. We look at how species (humans included)
cope with extremes (light, shade, drought, floods, lousy soils, etc.).
We try to understand how so many species can coexist in a single place
(900 species of birds in Panamá and around 300 tree species in 50
hectares of forest). We are definitely interested by a lingering
question... why so many tropical trees (and why is their identification
such a hellish job)? Finally, we want to get a global picture of
tropical systems by unravelling the interdependencies that make
ecosystems go-round.
Enough
about questions, we need answers! Good research is backed by good
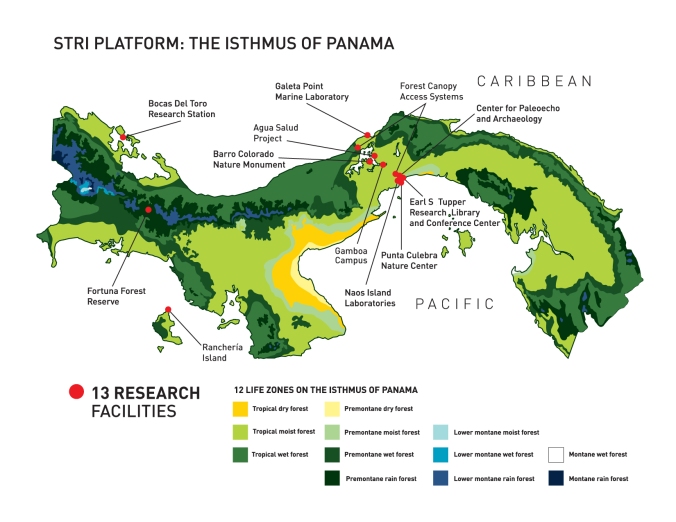
infrastructure. Luckily for us, you can’t really beat STRI. We have
access to 13 research facilities across the Isthmus of Panamá and here’s
a very brief description of each.
A map of all STRI research facilities in Panamá (Credit: STRI, http://stri.si.edu/reu/english/why_panama.php).
1) Earl S. Tupper Research, Library and Conference Center
This
set of buildings hosts most of the administrative units, a score of
laboratories equipped for all kinds of research, a herbarium, an insect
collection and a library comprising over 69,000 volumes centered on
tropical sciences. The old and rare books section is to die for... if
you like getting your hands on the drawings of 17th to 19th century
explorers.
The Earl S. Tupper Library holds over 69,000 volumes related to tropical sciences (Photo: Nicolas Chatel-Launay).
2) Center for Tropical Paleoecology and Archaeology (CTPA)
If
you dig fossils, that’s the place you want to be. Specialized in
geology, geography and archaeology, scientists working here try to
unravel the distant past, from giant (and thankfully extinct) snake
species to the processes that explain why North and South America became
one land mass three million years ago. Scientists from CTPA are
currently using the Canal expansion project as a way to dig further into
Panama’s past.
3) NAOS Island Laboratories
Located
at the Pacific entrance of the Canal, this research facility has a
state of the art molecular and genetics laboratory. It also has all you
need to keep oceanic critters alive for research. People here specialise
in Pacific oceanography and paleontology.
4) Galeta Point Marine Laboratory
NAOS’s
counterpart, this research facility is located at the Caribbean
entrance of the Canal. It is best known for research on the effects of
oil spills and on mangrove systems.
A view of one of the numerous coral reefs neighboring the Bocas Del Toro Research Station (Photo: Nicolas Chatel-Launay).
5) Bocas Del Toro Research Station
Located
in the Bocas Del Toro Archipelago, this station hosts scientists who
work on coral reefs, lagoon systems and lowland tropical forests. As it
is located on the Caribbean side, in the middle of a cultural melting
pot between Asia, Africa and the Americas, it is also a research hub on
human sociality.
6) Rancheria Island
Located
on a Pacific Island, this research station is in the middle of the
Eastern Pacific Ocean’s largest concentration of coral reefs. It is the
Pacific counterpart of Bocas Del Toro.
7) Punta Culebra Nature Center
Located
on a Pacific Island, this center focuses on public awareness and
outreach. Scientists try to test education strategies in order to better
transmit knowledge to the coming generations.
The Fortuna Forest Reserve lets scientists work in a unique ecosystem... cloud forest (Photo: Nicolas Chatel-Launay).
8) Fortuna Field Station
Fortuna
Forest Reserve is 1,200 meters (4,000 feet) up in the mountains and
lets scientists study a particularly interesting tropical ecosystem... a
cloud forest. I can tell you that the sun is rare out there, and it’s
constantly wet. Some areas of the reserve receive 12 meters of rain a
year (and have less than 30 rain-free days yearly).
A clear night sky in Fortuna is a rare event, less than 30 days a year are rainless (Photo: Nicolas Chatel-Launay).
9) Agua Salud
This
project, located within the Panamá Canal watershed covers 300,000
hectares. Scientists involved in this long-term study try to test the
best reforestation strategies and how different techniques can be used
to store carbon, control devastating floods, or improve soil
fertility... all without banning agriculture. People here try to get to
an optimal land-use strategy for the tropics.
10) Forest Canopy Access Systems
People
at STRI are all smart. But some have exceptionally smart ideas. Two
construction cranes were permanently installed in the rainforest on both
the Pacific and Caribbean sides so that scientists could easily access
the forest canopy. Wonder how we could get this close to a mommy sloth
and its baby in the posts from
Scott,
Librada and Flor? Yup, we were in a crane.
11) Gamboa Campus
Here
we are! this is the main base our group used for the Tropical Biology
Field Course 2015. Gamboa Campus is located at the dead center of the
Panamá Canal, and has a suite of laboratories. Also, a lot of
specialized research happens here. There is a system of “pods” to grow
plants in different temperature and atmospheric conditions to unravel
the effects climate change might have in the tropics. There are flight
cages that bats call home and where their behaviour is finely analyzed.
And there is Pipeline road, a well-known spot for anyone interested in
birds (See
Elise’s post on the IGERT-NEO blog).
Among all our activities in Gamboa, bat trapping was certainly one of the most interesting (Photo: Nicolas Chatel-Launay).
12) Barro Colorado Nature Monument (BCI)
The
Crown Jewel! Barro Colorado is an island, surrounded by three
peninsulas, all protected by the Panamanian government and the
Smithsonian Institution. Only research can go on here. With its 5,400
hectares, it is the oldest STRI facility, first occupied in 1924. The
island itself is a no-touch zone. You can measure and observe, but you
can’t change anything. The peninsulas are used for experiments, as in...
what happens if you kill all lianas in a forest? Do the trees grow
better? Or again, what happens if you change the nutrient regimes by
dumping tons of fertilisers?
A view of the main buildings on BCI island (Photo: Nicolas Chatel-Launay).
13) Center for Tropical Forest Science (CTFS)
Located
on BCI Island and founded in 1980, this 50 hectares forest plot gave us
the most precious data set ever collected in tropical biology. Every
single tree stem larger than 1 cm (there are roughly 200,000 of them),
is identified to species, measured, and recensused every five years. The
same goes for lianas, and many groups of shrubs. We also have precise
soil composition data all over the plot. We have mammal, bird and insect
inventories for the area. Many mammals and birds even have radio
collars; we can track their every movement in the forest. Basically, we
can have lots of fun with lots of data. Not only is the 50-hectare plot
an awesome dataset, it had children. CTFS plots are now all over the
Americas, Africa, Asia, Europe, and Oceania. People there collect data
in the same manner, using the same protocol. This way, we can compare
forests through space and through time, precisely, individual by
individual, all over the world. Imagine what questions you can explore
with that.
So
here we are! This was a small overview of what we do, and where we do
it. STRI is composed of biologists, archaeologists, anthropologists,
geographers, and specialists of other fields trying to answer one
question. What makes the tropics tick? And if you’re jealous, well don’t
be. You are welcome to join in this adventure.
--
Nicolas Chatel-Launay
























_male.jpg)